Project Description
Regional Training Center for Health Research Contribution towards evidence-based disease Control Policy and practice in South Asia by improving the activity of reasearch for diseases in South East Asia
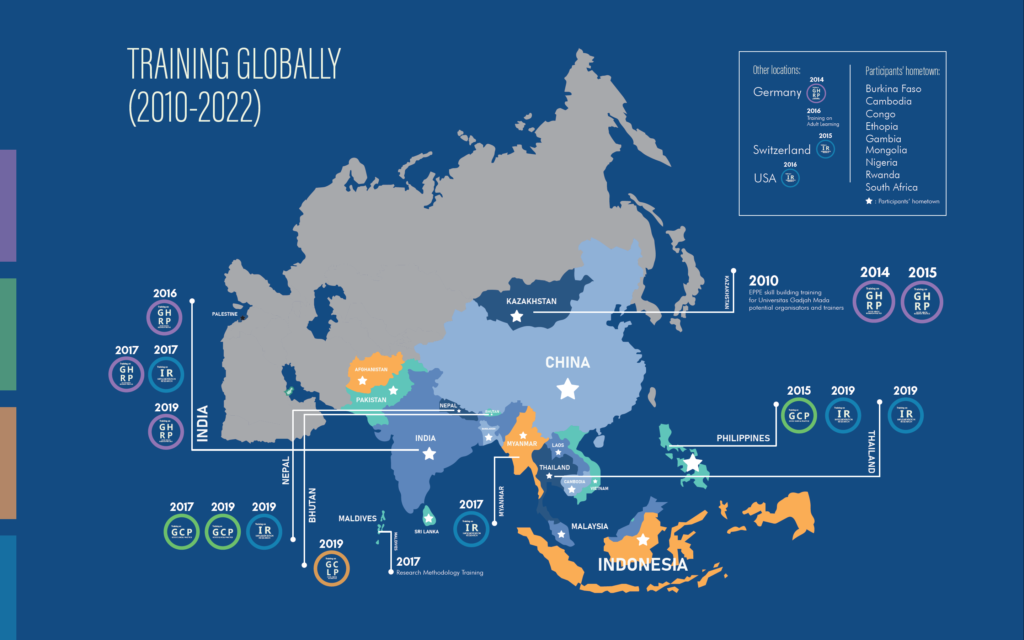
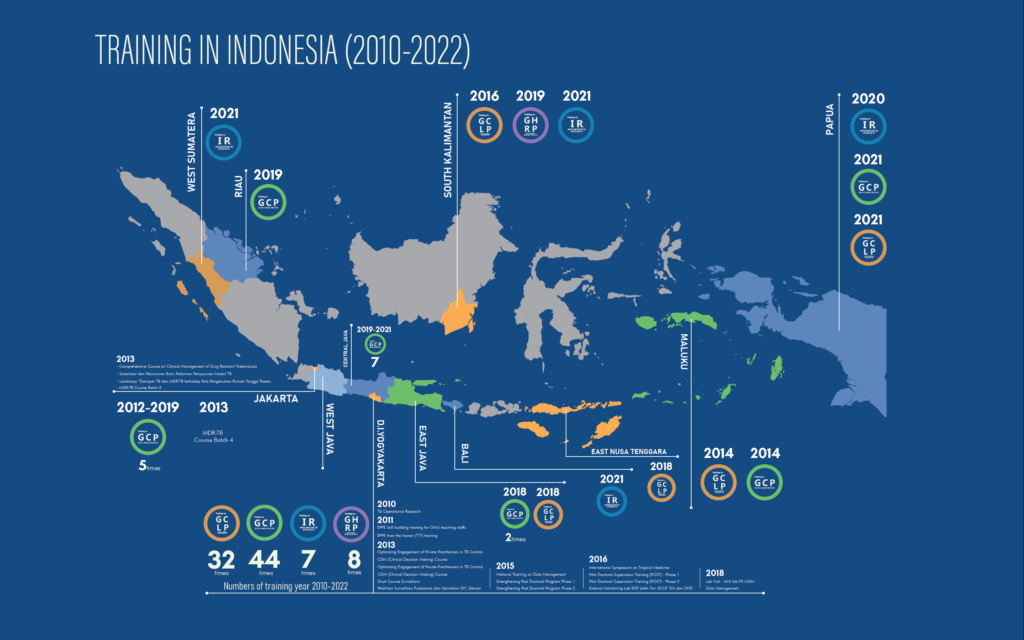
Since 2011, the Central for Tropical Medicine has collaborated with TDR to provide various types of training. Including Good Clinical Laboratory Practice (GCLP), Good Clinical Practice (GCP), Good Health Research Practice (GHRP) and Implementation Research (IR). Let’s meet them all.
According to the website of the Regional Training Center (retrac.fk.ugm.ac.id), “A guideline for using laboratory samples in clinical studies is known as Good Clinical Laboratory Practice. For many years, it has been widely acknowledged on a global scale that clinical laboratories require appropriate standards to guide good practices.” ” Good Clinical Practice, on the other hand, is “an international ethical and scientific standard for designing, conducting, analyzing, and reporting clinical trials involving human subjects’ participation.”
What about Good Health Research Practice? “It is a training course expected to help researchers conduct human health research.” “With IR has been defined in various ways by different institutions, common interpretations focus on the systematic approach to understanding and addressing barriers to effective and quality implementation of health interventions, strategies and policies.”
Funding

Collaborators

Duration
Principal Investigator
Yodi Mahendradhata

The Project Team


